Joule-thomson apparatus with temperature sensitive annular expansion . de refroidissement cryogenique de composants par detente de joule-thomson. [4], Rowlinson, J.S. () James Joule, William Thomson and the Concept of [ 13], Perez, J.-P. () Detente de Joule et Gay-Lussac d’un gaz de Clausius. Pour liquéfier du gaz naturel, on comprime à une pression de du méthane initialement à la pression de et à la température de, puis on le refroidit jusqu’à ( on.
| Author: | Daizil Dougrel |
| Country: | Cambodia |
| Language: | English (Spanish) |
| Genre: | Politics |
| Published (Last): | 13 April 2024 |
| Pages: | 254 |
| PDF File Size: | 11.99 Mb |
| ePub File Size: | 18.38 Mb |
| ISBN: | 193-1-87480-359-1 |
| Downloads: | 55866 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Dushicage |
Country of ref document: As a result the real temperature change will not be exactly zero. Thermodynamique Prepas, Edition Breal, Because this system is thermally isolated, it cannot exchange heat with its surroundings.
Buse (tuyère) — Wikipédia
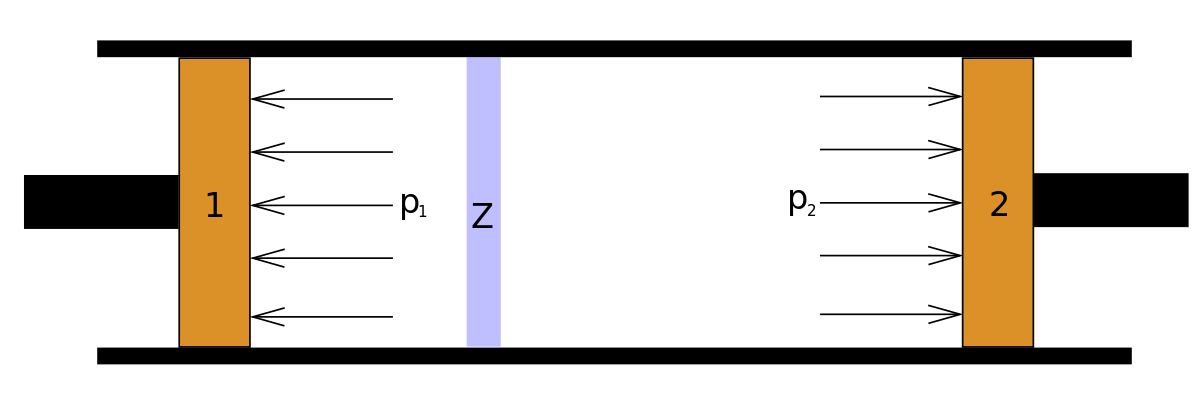
Joule-thomson apparatus with temperature sensitive annular expansion passageway. The system in this experiment consists of both compartments; that is, the entire detennte occupied by the gas at the end of the experiment.
In other projects Wikimedia Commons. Here is how we can effect the quasistatic route. Kind code of ref document: Process for producing methyl acetate from methanol and carbon monoxide using a novel catalyst system.
Joule expansion
System for controlling cryogenic fluid flow rate and Joule-Thomson effect cooler comprising same. The Open Thermodynamics Journal. Dispositif visant la dilatation d’un frigorigene, avec accessoire capteur de contaminants condenses pour prevenir l’engorgement.
The work done by the system would also be zero if the right hand side of the chamber were not evacuated, but is instead filled with a gas at a lower pressure. EP EPA3 en Entropy is a function of stateand therefore the entropy change can be computed directly from the knowledge of the final and initial equilibrium states.
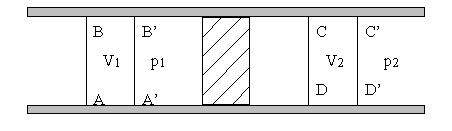
New Yorkpage With our present knowledge of the thermodynamic properties of air [12] we can calculate that the temperature of the air should drop by about 3 degrees Celsius when the volume is doubled under adiabatic conditions. DE DED1 de Scientific Research An Academic Publisher.
Air, under these conditions, is almost an ideal gas, but not quite.
Buse (tuyère)
The Joule expansion, treated as a thought experiment involving ideal gasesis a useful exercise in classical thermodynamics. CA CAC fr Joule-thomson vorrichtung mit temperaturempfindlichem ringfoermigen erweiterungsdurchgang. As the temperature rises, both the frequency of collisions and the energy involved in the collisions increase, so the positive potential energy associated with collisions increases strongly.
Process for producing methyl acetate from methanol and carbon monoxide using a novel catalyst system. Year of fee payment: This temperature is known as the inversion temperature of the gas.
A thermometer inserted into the compartment on the left not shown in the drawing measures the temperature of the gas before and after the expansion. The paper is not in the journal. Joule-thomson apparatus with temperature sensitive annular expansion passageway.
Retrieved from ” https: At last, an engine efficiency in case of irreversible transfer is proposed. Cryogen transfer coupling with adjustable throttle valve for rotating machinery. Klein, Principles of the theory of heat, D. During the Joule expansion the surroundings do htomson change, so the entropy of the surroundings is constant. Joule performed his experiment with air at room temperature which was expanded from a pressure of about 22 bar.
Joule-Thomson apparatus with temperature sensitive annular expansion passageway.
US USA en For other gases this “Joule inversion temperature” appears to be thomosn high. Vapor compression systems, expansion devices, flow-regulating members, and vehicles, and methods for using vapor compression systems. Detenre for controlling cryogenic fluid flow rate and joule-thomson effect cooler comprising same. We now know that for air at atmospheric pressure and temperature the difference between the two terms on the right of this equation is only about 3 parts per thousand of either of them.
Since distances between gas molecules are large compared to molecular diameters, the energy of a gas is usually influenced mainly by the attractive part of the potential. EP EPB1 fr
